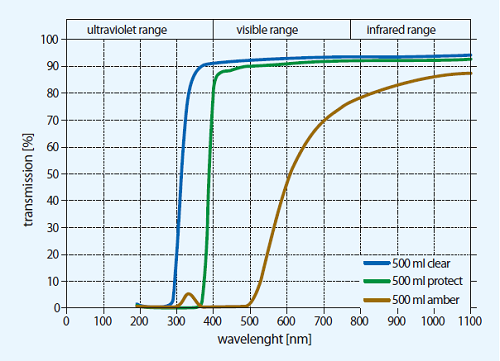
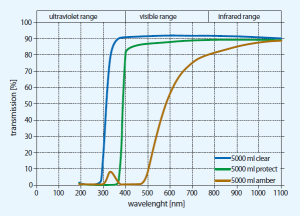
WHAT IS GLASS?
Glass is an inorganic mixture fused at high temperature which solidifies on cooling but does not crystallize. Its basic components, network formers and modifiers, are present in the common glasses in the form of oxides. Typical glass formers (network formers) are silicon dioxide (SiO2), boron trioxide (B2O3), phosphorus pentoxide (P2O5) and aluminium oxide (Al2O3). These substances are capable of absorbing (dissolving) metal oxides up to a certain proportion without losing their glassy character. This means that the incorporated oxides are not involved in the formation of the glass but modify certain physical properties of the structure of the glass as “network modifiers”. A large number of chemical substances have the property that they solidify from the molten state into a glassy state. The formation of glass depends on its cooling rate and a necessary prerequisite is the existence of mixed types of bond (covalent bonds and ionic bonds) between the atoms or groups of atoms. As a result, glass forming products show a strong tendency whilst still in the molten state towards amorphous three-dimensional networking though polymerisation. Crystals are formed when the individual atoms form a regular three-dimensional arrangement in what is known as a “crystal lattice” as soon as the particular substance changes from the liquid to the solid state. Glass, however, forms a largely amorphous “network” when it cools down from the molten state. The components mainly involved in the formation of the glass are therefore described as “network formers”. The glass forming molecules in this network can incorporate ions that open up the network at certain points, changing its structure and thus the properties of the glass. They are therefore called “network modifiers”.
WHAT IS DURAN®?
The special features of DURAN®
Very high chemical resistance, nearly inert behaviour, a high usage temperature, minimal thermal expansion and the resultant high resistance to thermal shock are its most significant properties of boro 3.3. This optimum physical and chemical performance makes DURAN® borosilicate glass the ideal material for use in the laboratory and for the manufacture of chemical apparatus used in large-scale industrial plant. It is also widely used on an industrial scale in all other application areas in which extreme heat resistance, resistance to thermal shock, mechanical strength and exceptional chemical resistance are required.
Chemical composition of DURAN®
DURAN® has the following approximate composition:
| 81 |
% by weight |
SiO2 |
| 13 |
% by weight |
B2O3 |
| 4 |
% by weight |
Na2O / K2O |
| 2 |
% by weight |
Al2 |
DURAN® properties are specified in DIN ISO 3585.In contrast to other borosilicate 3.3 glasses, DURAN® is notable for its highly consistent, technically reproducible quality.
Chemical properties
The chemical resistance especially of DURAN® glass is more comprehensive than that of all other known materials. DURAN® borosilicate glass is highly resistant to water, acids, saline solutions, organic substances and also halogens such as chlorine and bromine. Its resistance to alkali is also relatively good. Only hydrofluoric acid, boiling phosphoric acid and strong alkalis cause appreciable surface removal of the glass (glass corrosion) at elevated temperatures (> 100 °C). Due to the nearly inert behaviour, there are no interactions (e.g. ion exchange) between medium and glass and any spurious influence on experiments is thereby effectively excluded.
Hydrolytic resistance
DURAN® corresponds to Class 1 of the glasses that are divided into a total of 5 hydrolytic resistance classes in accordance with ISO 719 (98 °C). The amount of Na2O/g glass grain leached out after 1 hour in water at 98 °C is measured. For DURAN® the quantity of Na2O leached out is less than 31 μg/g of glass grain. DURAN® also corresponds to Class 1 of the glasses divided into a total of 3 hydrolytic resistance classes in accordance with ISO 720: (121 °C). The quantity of Na2O leached out after 1 hour in water at 121 °C is less than 62 μg/g of glass grain. Due to its good hydrolytic resistance, DURAN® meets the requirements of the USP, JP and EP for a neutral glass that corresponds to glass type 1. It can therefore be used in an almost unrestricted way in pharmaceutical applications and in contact with foodstuffs.
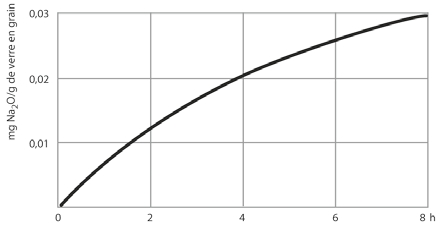 |
Hydrolytic attack on DURAN®
as a function of time (h) |
Acid resistance
DURAN® corresponds to Class 1 of the glasses divided into 4 acid classes in accordance with DIN 12116. As the surface removal after boiling for 6 hours in normal HCl is less than 0.7 mg/100 cm2, DURAN® is classed as acid resistant borosilicate glass. The quantity of alkaline metal oxides leached out in accordance with ISO 1776 is less than 100 μg Na2O/100 cm2.
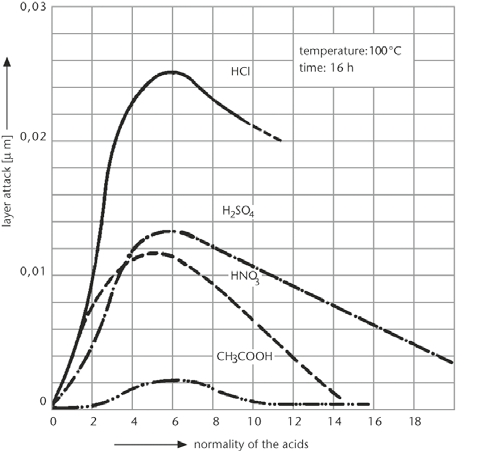 |
Acid attack on DURAN®
as a function of acid concentration |
Alkali resistance
DURAN® corresponds to Class 2 of the glasses divided into 3 alkali classes in accordance with DIN ISO 695. The surface erosion after 3 hours boiling in a mixture of equal volume fractions of sodium hydroxide solution (concentration 1 mol/l) and sodium carbonate solution (concentration 0.5 mol/l) is only 134 mg/100 cm2.
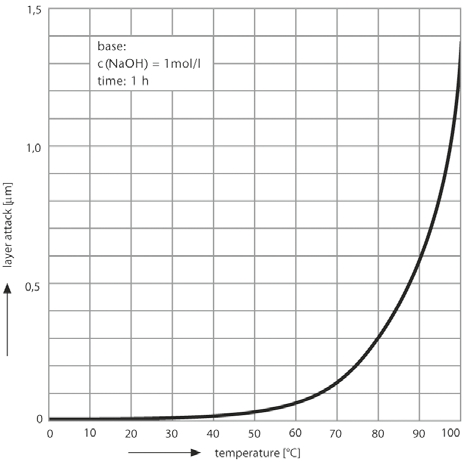 |
Alkali attack on DURAN®
as a function of temperature (°C) |
Overview of the chemical properties of technical glasses
| Description |
Chemical resistance class |
|
Hydrolytic resistance |
Acid resistance |
Alkali resistance |
|
DIN ISO 719 |
DIN 12 116 |
ISO 695 |
| DURAN® (boro 3.3) |
1 |
1 |
2 |
| FIOLAX® (boro 4,9) |
1 |
1 |
2 |
| Soda-lime-glass |
3 |
1 |
2 |
| SBW |
1 |
1 |
1 |